
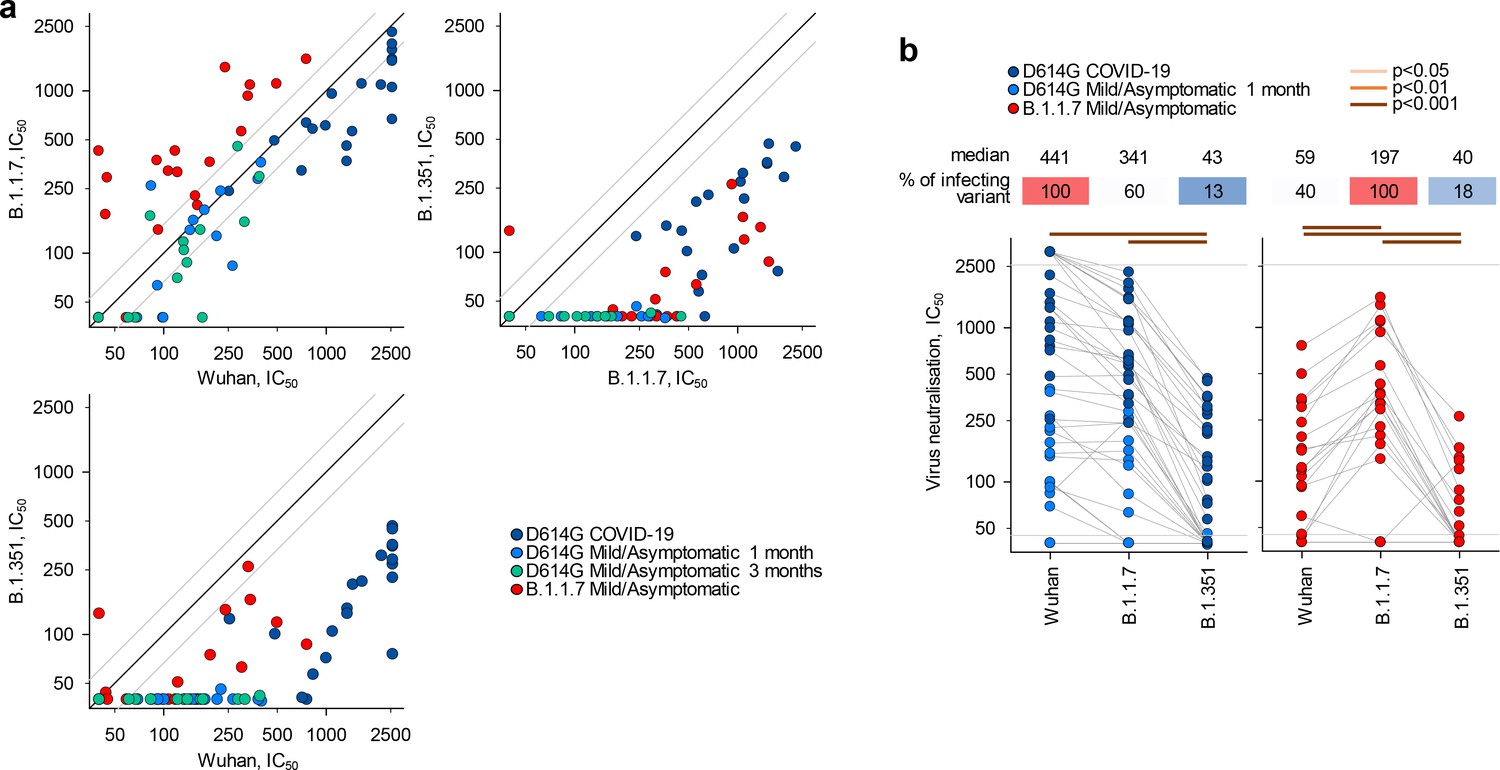
See also Figures S5 and S6.Īhmed S.F., Quadeer A.A., McKay M.R. 154C and 240C showed only partial neutralization at the highest concentration tested (1:10 dilution), whereas 341C, 540C, and CR3022 failed to reliably neutralize pseudotyped virus at this dilution. The concentration of all monoclonal antibody stocks was 1 mg/mL. 1v6 is positive control from COVID-19 patient convalescent serum collected at day 14. EC 50 values represent triplicate experiments (n = 3). (E) Neutralization assay 50% neutralization values against live SARS-CoV-2 by focus-forming assay and SARS-CoV-2 spike pseudotyped lentivirus by fluorescence microscopy. (D) Summary of quantified binding kinetics of Spike monoclonal antibodies from BLI experiment.
Curve fitting was performed using 1:1 binding model in ForteBio Analysis HT 10.0 software. Negative binding curves for 341C and 540C shown in Figure S5. Streptavidin biosensors were coated with biotinylated RBD, then blocked with 1 μM D-Biotin in kinetics buffer. All rights reserved.īinding kinetics and functional testing of Spike-specific antibodies against the RBD of the SARS-CoV-2 (A–C) Biolayer interferometry curves for CR3022, 154C, and 240C with 3-fold dilutions. Second, we provide empirical evidence of the high propensity for antibody cross-reactivity between distinct strains of human coronaviruses, which is critical information for designing diagnostic and vaccine strategies for COVID-19.ĬOVID-19 CSARS-CoV-2 antibody cross-reactivity envelope membrane nucleocapsid spike.Ĭopyright © 2021 The Author(s). First, we establish a set of antibodies with known reactivity to both SARS-CoV and SARS-CoV-2, which will allow further study of both viruses. The implications of our work are two-fold. Although nearly all of the examined SARS-CoV antibodies display some level of reactivity to SARS-CoV-2, we find only partial cross-neutralization for the spike antibodies. We extensively characterize a selection of 10 antibodies covering all of the SARS-CoV structural proteins: spike, membrane, nucleocapsid, and envelope. Here, we investigate the cross-reactivity of antibodies raised against the first severe acute respiratory syndrome coronavirus (SARS-CoV) for their reactivity toward SARS-CoV-2. The content of the study report is based upon FDA recommendations and includes a signature page, executive summary, methods, materials, scoring table results, analysis, peer review and conclusions.In the ongoing coronavirus disease 2019 (COVID-19) pandemic, there remain unanswered questions regarding the nature and significance of the humoral immune response toward other coronavirus infections. Prior to staining, we undertake an assessment of tissue integrity to confirm that each tissue within the panel has been handled correctly with no areas of necrosis or autolysis and optimal autogenicity and morphology is present.Įvaluation and scoring of the stained tissue panel by an experienced pathologist is highly beneficial – not only to ensure accurate assessment of target binding and distribution, but also to convey credibility during the regulatory submission process.Īll TCR studies at Propath are conducted under GLP conditions and under the direction of an experienced Study Director, are evaluated by an experienced pathologist (with or without a peer review), and undergo a study-specific QA audit to confirm GLP compliance. Non-human tissues in addition to human tissues are often tested. The panel may be extended to include additional organs depending on the specific requirements of a study or to conform with EMA recommendations. This conforms with the recommendations in the FDA document, “Points to Consider in the Manufacture and Testing of Monoclonal Antibody Products for Human Use”. Within this phase, we assess cross-reactivity of the test article and its isotype control at three different dilutions, across triplicate specimens on a minimum of 33 human organs.
ANTIBODY CROSS REACTIVITY FULL
Phase 2: TCR study in a full panel of tissues under GLP conditions


 0 kommentar(er)
0 kommentar(er)
